Leukemia is defined by the presence of more than 25% malignant hematopoietic cells (blasts) in the bone marrow aspirate. Acute lymphoblastic leukemia/lymphoblastic lymphoma (ALL/LBL) is the most common childhood malignancy. Leukemia and lymphoma are overlapping clinical presentations of the same disease.
Age
- The peak age at onset is 4 years
- 85% of patients are diagnosed between ages 2 and 10 years.
- More common among boys than girls
- Children with Down syndrome have a 14-fold increase in the overall rate of leukemia.
Classification
- B-lineage
- T-lineage
- Uncommon variants (NK-lineage, early T progenitor ALL)
WHO Classification
WHO classification on basis of IMMUNOPHENOTYPE
B Lymphoblastic leukemia
Lymphoblast (TDT+) commonly expressing CD10(90%), CD 19 , CD 20, CD 79a & HLA-DR
Types –
- Common precursor B Cell ALL –
- CD 10 (+ve)
- No surface or cytoplasmic Ig
- 75% best prognosis
- Pre B ALL –
- Cytoplasmic Ig present as more closer to maturity
- Pro B ALL –
- CD 10 (-ve)
- No surface or cytoplasmic Ig
- 10% most common in INFANTS
T Lymphoblastic Leukemia
- NOTCH gene mutation 50% of all T- ALL cases
- t(5 ;14), (q35; q32) – 20% poor prognosis, treatment failure
- Early precursor – CD 1a,CD 8 has poorer prognosis
Clinical presentation
Presenting complaints of patients with ALL include those related to decreased bone marrow production of red blood cells (RBCs), white blood cells (WBCs), or platelets and to leukemic infiltration of extramedullary (outside bone marrow) sites –
- Fatigue, bleeding/ bruising, Infection
- B Symptoms – Fever, Night Sweats, Weight Loss(>67%) often present but mild.
- Lymphadenopathy/Hepatomegaly/Splenomegaly(50%) indicative of extramedullary spread
- Muscular Pain classically nocturnal bone pain(25%)
- Headache/Neuropathy(5%) commonly from meningeal involvement and increased ICP
Rare but important
- Testicular enlargement classically pain less
- Mediastinal mass classically thymic mass in teenager with T-CELL ALL
- Massive Mediastinal Adenopathy leading to airway obstruction
- Renal Failure due to leukemic infiltration of renal parenchyma
- DIC
Physical examination
- Pallor, petechiae, and purpura
- Hepatomegaly and/or splenomegaly occur in over 60% of patients
- Lymphadenopathy is common, either localized or generalized to cervical, axillary, and inguinal regions.
- A mediastinal mass, which is most often associated with T-ALL/LBL
Laboratory Findings
- 95% of patients with ALL have a decrease in at least one cell type (single cytopenia): neutropenia, thrombocytopenia, or anemia.
- in 50% of patients WBC count is low or normal (= 10,000/μL), but the differential shows neutropenia (absolute neutrophil count < 1000/μL) along with a small percentage of blasts amid normal lymphocytes
- In 30% of patients the WBC count is between 10,000/μL and 50,000/μL
- In 20% of patients it is over 50,000/μL, occasionally higher than 300,000/μL.
- Blasts are usually readily identifiable on peripheral blood smears from patients with elevated WBC counts.
- Peripheral blood smears also show abnormalities in RBCs, such as teardrops
- Most patients with ALL have decreased platelet counts (< 150,000/μL) and decreased hemoglobin (< 11 g/dL) at diagnosis
- Serum chemistries, particularly uric acid and lactate dehydrogenase (LDH), are often elevated at diagnosis as a result of cell breakdown.
Histopathology
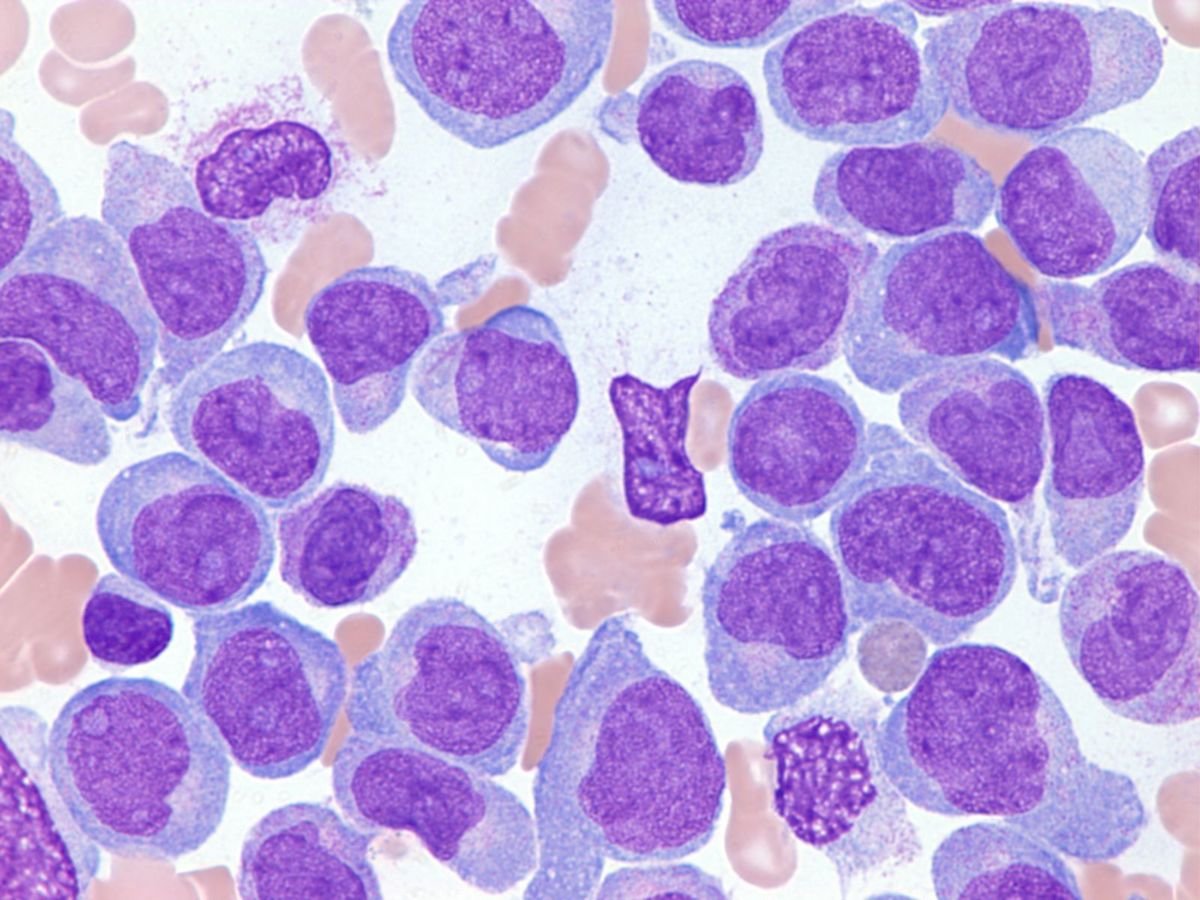
Peripheral blood smear of acute lymphoblastic leukemia –

Acute lymphoblastic leukemia. Peripheral blood smear shows small, uniform blasts with scant cytoplasm and inconspicuous nucleoli. Wright-Giemsa, 100x magnification.
Bone marrow biopsy of a case of B cell acute lymphoblastic leukemia-
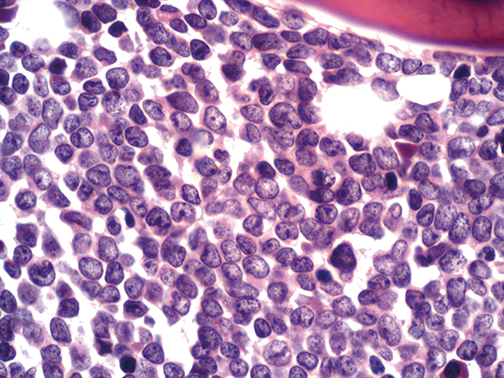
Increase cellularity from blast M1<5%,M2 (5-20%),M3 >25%
Flow Cytometry
- Immunophenotyping of ALL blasts by flow cytometry helps distinguish precursor B-cell ALL from T-cell ALL or AML
- The anti-CD45 antibody is the most used reagent for immunophenotyping acute leukemias. The analysis of CD45/SSC dot plot is mandatory when analyzing leukemic cells because this graph allows to isolate blast cells from other hematopoietic cell types
- Diagnostic immunophenotyped of ALL/LBL requires confirmation of lymphoid lineage and exclusion of myeloid lineage by flow cytometry
- Blasts in pre B-cell ALL can be initially identified using a SSC vs CD45 plot. These blasts have low SSC (many times smaller than normal lymphocytes) and dim to negative CD45 for B-cell ALL. This differs from blasts in AML where the SSC is generally higher.
- B-lineage lymphoblasts are almost always positive for the B cell markers CD19, cytoplasmic CD79a, and cytoplasmic CD22
- B lymphoblasts must be negative for CD3 (T cell antigen) and negative for myeloperoxidase (MPO)
- Although there is no consensus regarding a minimal proportion of lymphoblasts in bone marrow, the diagnosis of B-ALL/LBL should be avoided when there are <20 percent lymphoblasts.

Above image is B-ALL Immunophenotyping
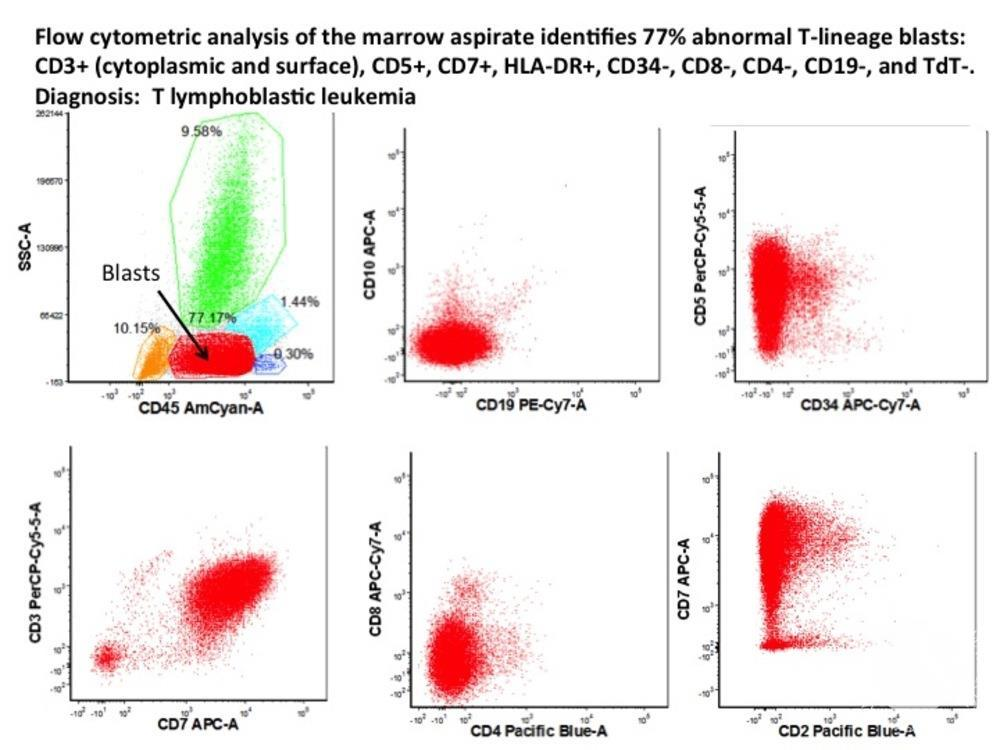
Above image is T-ALL Immunophenotyping
Cytochemistry
Myeloperoxidase (MPO) – By definition, MPO is negative in ALL/LBL, since peroxidase is considered a lineage defining myeloid marker.
Periodic acid-Schiff (PAS) – Lymphoblasts may reveal coarse (“chunky”) PAS-positive granules.
Nonspecific esterase (NSE) – Staining is usually absent
Differential Diagnosis
1. Burkitt lymphoma –
Burkitt lymphoma (BL) is a highly aggressive B cell non-Hodgkin lymphoma that can present with a rapidly growing tumor mass (eg, jaw or abdominal mass) and/or a leukemia that can have substantial clinical and morphologic overlap with ALL/LBL. BL histology typically reveals highly proliferative monomorphic medium-sized cells with basophilic cytoplasm (often with a “starry sky” appearance) that are generally larger than lymphoblasts of ALL/LBL
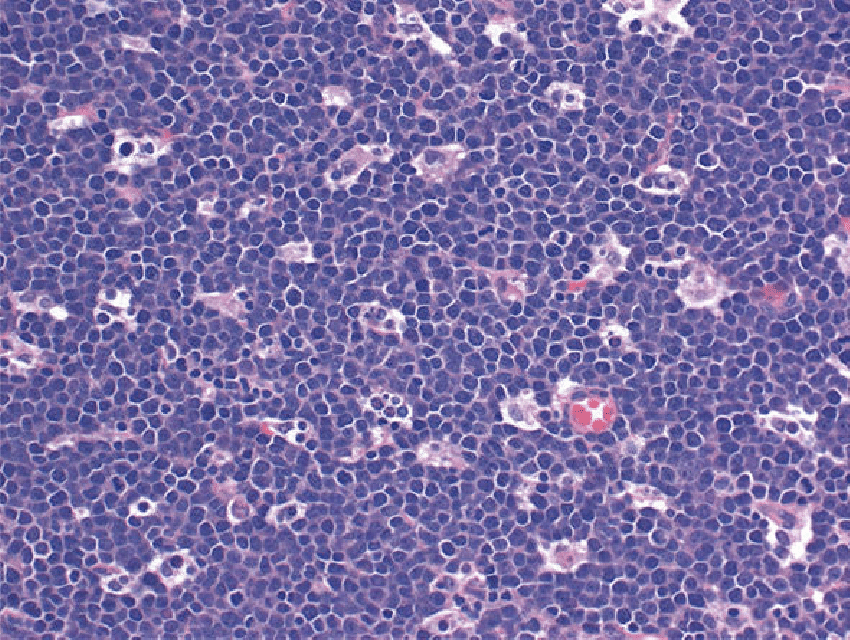
Burkitt lymphoma. The “starry-sky” appearance with numerous tingible body macrophages. Scattered mitotic figures are seen. The cells are medium in size with scant cytoplasm and squared-off cytoplasmic borders
2. Acute myeloid leukemia (AML) –
Myeloblasts of AML are typically immature cells with large nuclei, prominent nucleoli, and a variable amount of pale blue cytoplasm (sometimes with faint granulation and/or Auer rods). AML cells generally stain for myeloperoxidase (MPO) or lysozyme and are typically negative for B and T cell antigens and TdT
3. Acute undifferentiated leukemia (AUL)
AUL blast cells are morphologically bland and generally indistinguishable from those of B-ALL/LBL
4. Mixed phenotype acute leukemia (MPAL)
Diagnosis of MPAL requires demonstration of both myeloid and lymphoid defining markers by flow cytometry and/or immunohistochemistry
5. Chronic myeloid leukemia (CML)
CML is typically manifested as an expanded population of myeloid cells at various stages of differentiation with the characteristic t(9;22) (Philadelphia chromosome) with BCR-ABL1 rearrangement that may be accompanied by splenomegaly and/or constitutional symptoms.

6. Aplastic anemia
Aplastic anemia (AA) may present in children as pancytopenia with reticulocytopenia (frequently <10,000/microL) and profoundly hypocellular bone marrow with a decrease in all hematopoietic elements. In contrast to ALL/LBL, there is no infiltrate of malignant cells in AA instead, the marrow space is largely composed of fat cells and marrow stroma.
7. Small round blue cell tumors
In children, small round blue cell tumors, including Ewing sarcoma (ES) and peripheral primitive neuroectodermal tumor (PNET) may resemble B-ALL/LBL morphologically, but these disorders are distinguished by a uniform population of small, round, blue cells with hyperchromatic nuclei and scant cytoplasm; absence of B-lymphoid markers
8. Immune thrombocytopenia (ITP)
Is associated with isolated thrombocytopenia that can resemble ALL/LBL clinically. The diagnosis of ITP in a healthy-appearing child with a typical presentation (i.e sudden onset of a petechial rash or bruising with isolated thrombocytopenia) is straightforward.
Treatment
Specific Therapy
▶The first month of therapy consists of induction, at the end of which over 95% of patients exhibit remission on bone marrow aspirates by morphology. The drugs most commonly used in induction include oral prednisone or dexamethasone, intravenous vincristine, daunorubicin, intramuscular or intravenous asparaginase, and intrathecal methotrexate.
▶Consolidation is the second phase of treatment, during which intrathecal chemotherapy along with continued systemic therapy and sometimes cranial radiation therapy are given to kill lymphoblasts “hiding” in the meninges.
▶Several months of intensive chemotherapy follows consolidation, often referred to as intensification. This intensification has led to improved survival in pediatric ALL.
Maintenance therapy
▶Maintenance therapy can include daily oral mercaptopurine, weekly oral methotrexate, and, often monthly pulses of intravenous vincristine and oral prednisone or dexamethasone.
▶Intrathecal chemotherapy, either with methotrexate alone or combined with cytarabine and hydrocortisone, is usually given every 2–3 months