Classification Mycobacteria
- Mycobacterium tuberculosis complex – It is responsible for tuberculosis (TB) in man
- Mycobacterium leprae (Hansen’s bacilli) – It causes leprosy, characterized by anesthetic skin patches and lesions of peripheral nerves. If not treated, can lead to deformities affecting eyes, nose, hands and feet
- Nontuberculous mycobacteria (NTM) – These are a diverse group of mycobacteria.
- They are either saprophytic in nature, e.g. M. gordonae (from tap water)
- May be found as commensal (M. smegmatis in urine)
- Some of them can cause opportunistic human infections, e.g. M. kansasii
– TUBERCULOSIS –
Tuberculosis, caused by the M. tuberculosis complex, is one of the oldest diseases of mankind, and is a major cause of death worldwide. It usually affects the lungs, although other organs are also involved.
M. tuberculosis complex includes –
- M. tuberculosis : It is the most common species to cause TB in man
- M. bovis (bovine tubercle bacillus)
- Other members are less common human pathogens, such as M. africanum, M. microti, M. caprae, M. pinnipedii, M. canetti, M. suricate, M. orygis, M.mungi and the recently described dassie bacillus and chimpanzee bacillus.
Pathogenesis
Source of Infection
- Human (e.g. cases of pulmonary tuberculosis)
- Bovine source (e.g. consumption of unpasteurized infected milk)
Mode of Transmission
- Airborne – M. tuberculosis is mainly transmitted by inhalation of aerosols, generated while coughing, sneezing, or speaking of infected patients. They are tiny dry droplet nuclei (<5 μm size).
- Other modes of transmission are rare, such as –
- Inoculation – Transmission of infection through direct skin contact with an infected person is uncommon
- Ingestion – Swallowing of sputum (in infants)
- Consumption of unpasteurized (infected) milk.
Risk Factors
The risk factors favoring the transmission of infection include –
- Sputum positive patients
- Bacillary load – At least 104 bacilli/mL in sputum
- Adult patients with cavitary lesions in lung have more bacillary load in sputum (105–107 AFB/mL)
- Culture negative pulmonary TB and extrapulmonary TB patients are essentially noninfectious.
- Overcrowding in poorly ventilated rooms.
- Low cell mediated immunity
- Other comorbid conditions such as: Post-silicosis, post-transplantation (renal, cardiac), jejunoileal bypass, gas- trectomy, chronic renal failure/hemodialysis, diabetes, IV drug abuse, smoking, etc.
- Age: Late adolescence, early adulthood and elderly people
- Sex: Risk is higher in women at 25–34 years of age, while at older ages, men have a greater risk.
Clinical Manifestations
Tuberculosis (TB) is classified as pulmonary and extra- pulmonary forms.
- Pulmonary tuberculosis (PTB) – 60–90% of all cases of tuberculosis (TB).It is two types Primary and Post primary (secondary)
- Extrapulmonary Tuberculosis (EPTB) – EPTB results from hematogenous dissemination of tubercle bacilli to various organs

Laboratory Diagnosis
Diagnosis of active tuberculosis
1. Specimen Collection –
In PTB, two sputum samples are recommended –
- Spot sample (collected on the same day under supervision)
- Early morning sample (collected on the next day).
- Alternatively 2 spot samples at least one hour apart can be collected.
The extrapulmonary specimens vary depending on the site involved, which can be divided into two categories –
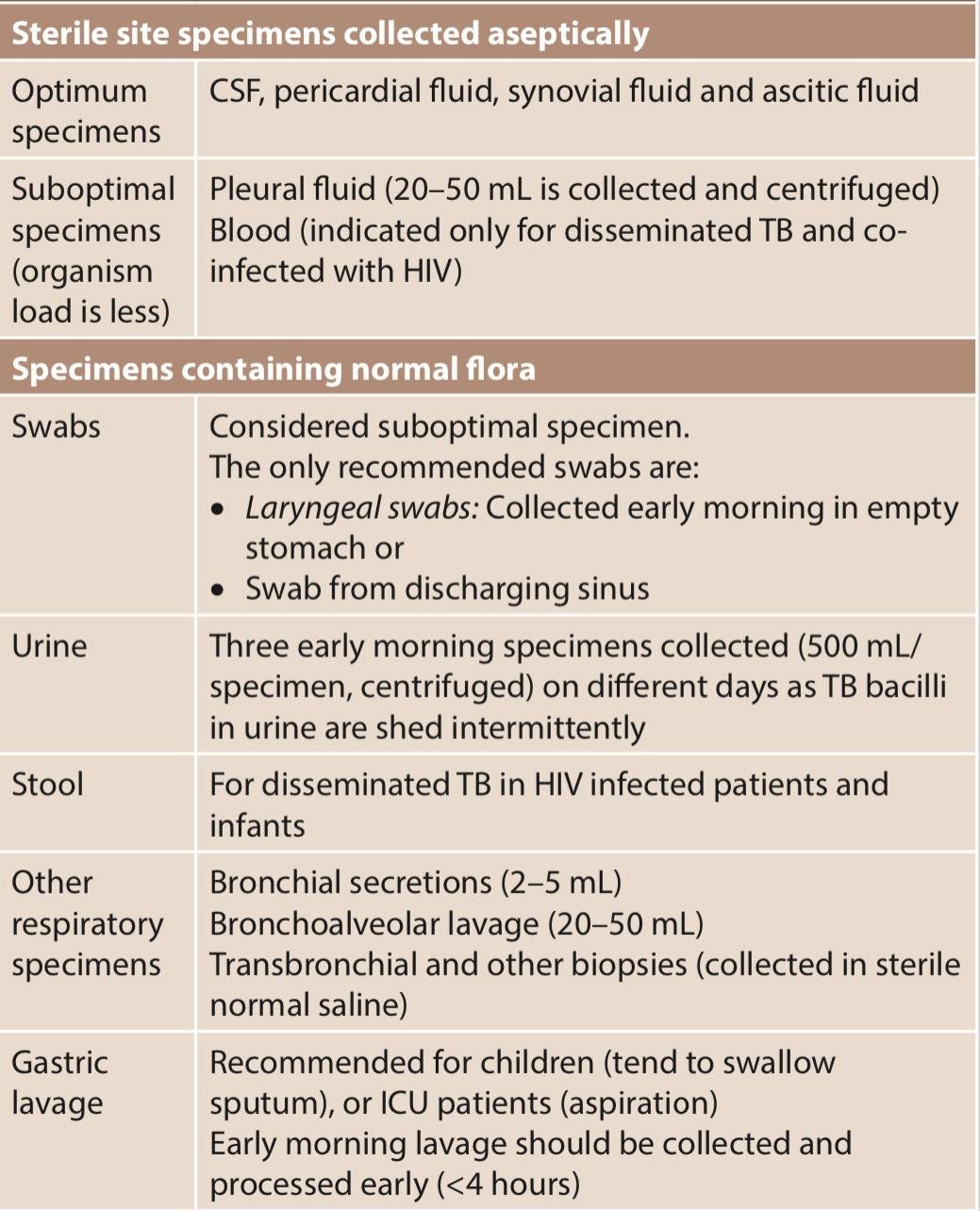 2. Digestion, decontamination and concentration of specimen –
2. Digestion, decontamination and concentration of specimen –
- Modified Petroff’s method (4% NaOH)
- NALC (N-acetyl-L-cysteine) + 2% NaOH
3. Direct microscopy by acid-fast staining
- Ziehl-Neelsen (ZN) technique
Smears are prepared from a thick mucopurulent part of sputum or with the sediment obtained after concentration. Optimum thickness of the smear can be assessed by placing the smear on printed matter. The print should be just readable through the smear. Then the smear is stained by acid-fast stain.
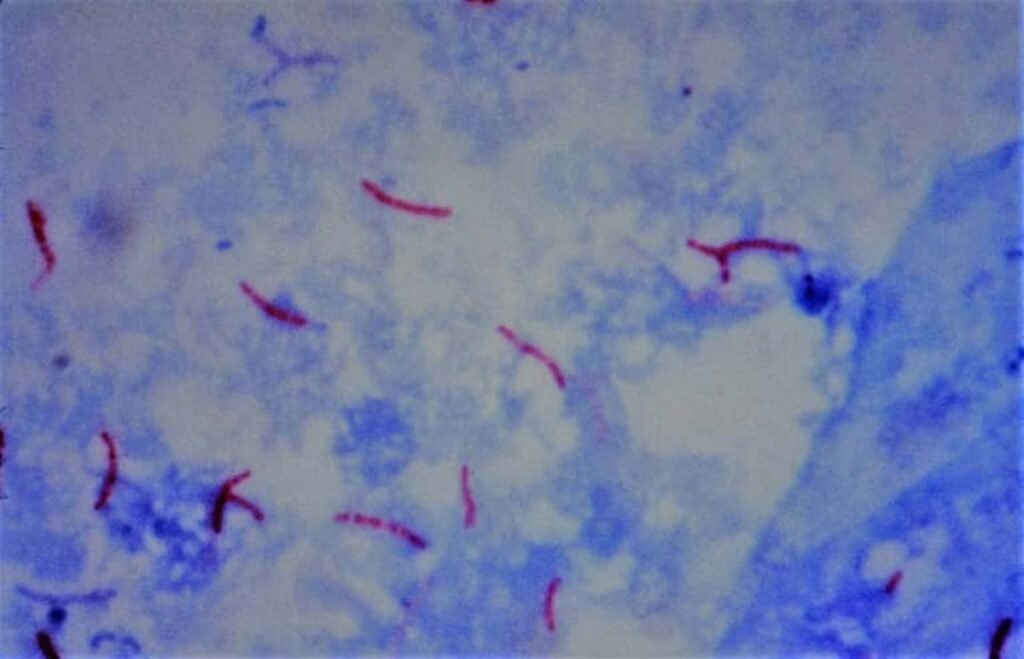
Interpretation –
- Negative result: At least 100 oil immersion fields should be examined for 10–15 minutes before giving a negative report
- Positive result: M. tuberculosis appears as long slender, beaded, less uniformly stained red colored acid-fast bacilli (AFB)

Presumptive diagnosis-
Microscopy provides only presumptive diagnosis. If typical beaded appearance is seen, then it should be reported as ‘acid-fast bacilli’ resembling M. tuberculosis are seen by smear microscopy by ZN stain.
- Advantages: Smear microscopy is rapid, easy to perform at peripheral laboratories and is cheaper
- Disadvantages: (i) Less sensitive than culture, (ii) low sensitivity with a detection limit of 10,000 bacilli/mL of sputum, (iii) It cannot determine the viability of bacilli
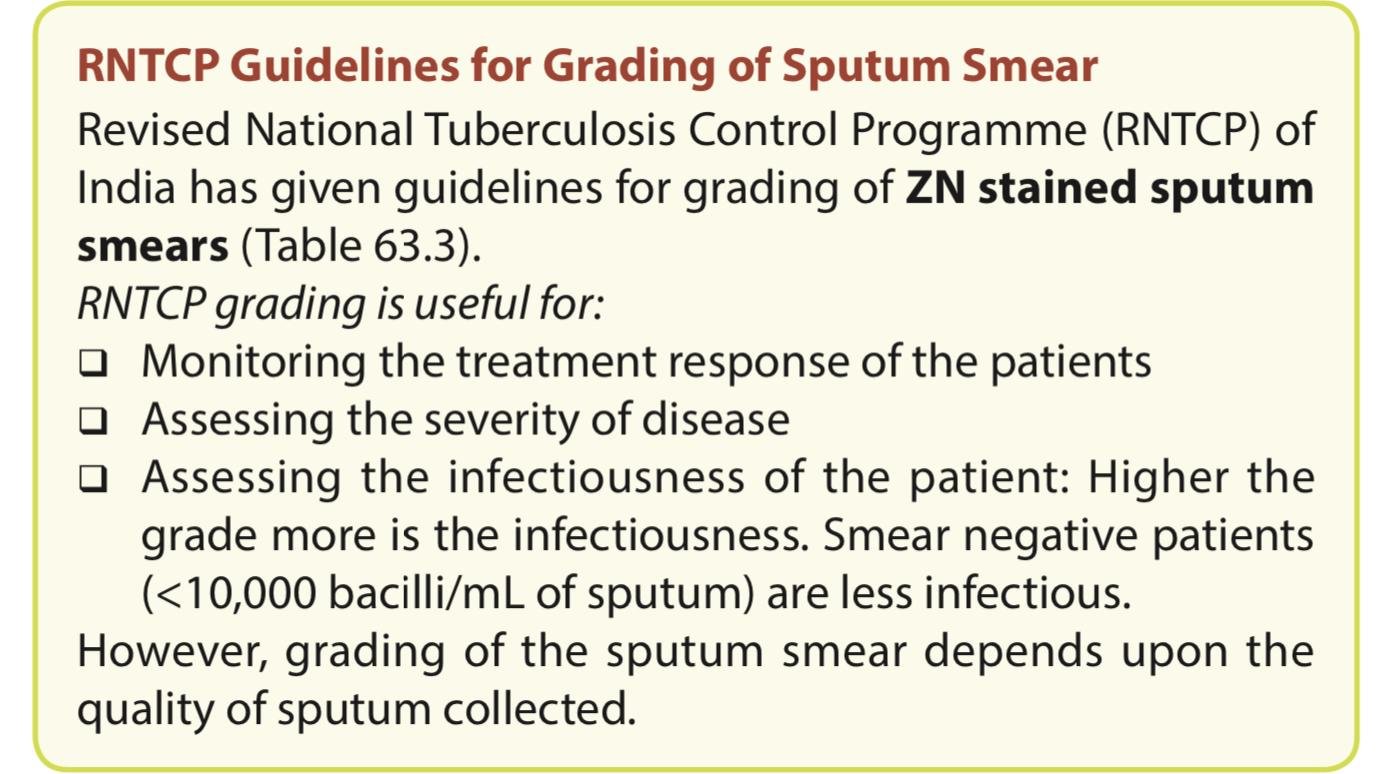
It is difficult to differentiate M. tuberculosis from saprophytic mycobacteria present in tap water or even as commensal in clinical samples such as gastric aspirate, and urine
- Kinyoun’s cold acid-fast staining
- Fluorescent (auramine) staining – It is more sensitive and smears can be screened more rapidly than ZN stain

4. Conventional culture media –
It takes 6–8 weeks
- Lowenstein Jensen (LJ) medium—shows rough, tough and buff colored colonies in 6–8 weeks

5.Automated culture methods –
It take 3–4 weeks
- MGIT system – Detects growth as well as resistance to antitubercular drugs
6.Culture identification –
- Automated identification – By MALDI-TOF
- MPT 64 antigen detection – By ICT
7.Molecular methods –
- PCR detecting IS6110 gene
- CBNAAT (GeneXpert) and Truenat – For identification and detection of resistance to rifampicin; has a turnaround time 2 hours
- Line probe assay (e.g. Genotype TB) – For identification and detection of resistance to 1st and 2nd line ATDs; has a turnaround time of 2–3 days.
Diagnosis of latent tuberculosis
Latent tuberculosis is diagnosed by demonstration of delayed or type IV hypersensitivity reaction against the tubercle bacilli antigens. Two methods are available,
- Tuberculin skin test
- IFN-γ release assay
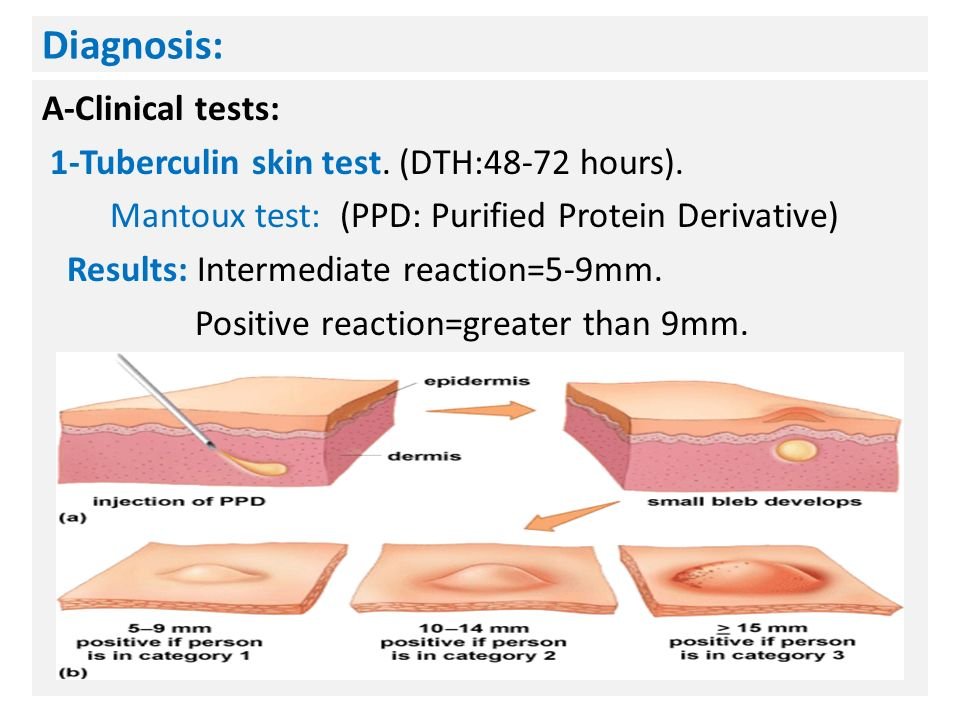
1. Tuberculin Skin Test (TST) –
- Antigens used – Purified protein derivative, known as PPD-23 for performing TST
- Dosage – Tuberculin unit (TU). One TU is equal to 0.00002 mg of PPD
- Procedure – Mantoux test is the most commonly employed method. 0.1 mL of PPD containing 1 TU is injected intradermally into the flexor surface of forearm
- Reading – It is taken after 48–72 hours. At the site of inoculation, an induration surrounded by erythema is produced. If the width of the induration is:
- ≥10 mm: Positive (tuberculin reactors)
- 6–9 mm: Equivocal/doubtful reaction
- <5 mm: Negative reaction.
- Interpretation of result in Adults –
- A Positive TST in adults only indicates present or past exposure with tubercle bacilli but does not confirm the presence of the active stage of the disease. Hence, it is only used as an epidemiological marker
- Prevalence of tuberculosis is calculated by counting all tuberculin reactors in a community
- Incidence Of Tuberculosis Calculated By Counting new converters to TST in a community.
- Interpretation of result in Children –
- A Positive test indicates active infection and is used as a diagnostic marker.
- False Positive: The test becomes positive after BCG vaccination (after 8–14 weeks), Nontuberculous mycobacteria infection.
- False Negative: The test may become negative in various conditions such as—early or advanced TB, miliary TB, decreased immunity (HIV-infected people).
2. Interferon Gamma Release Assay (IGRA)
- This uses highly specific M. tuberculosis antigens such as CFP10 (culture filtrate protein) and ESAT6 (early secreted antigenic target-6) – Both coded by RD1 genes.
- Procedure – In contrast to TST, it is an in vitro test.
- Sensitized T lymphocytes collected from suspected individuals are exposed to ESAT-6/CFP-10 antigens, which lead to release of high levels of IFN-γ from the T lymphocytes.
Treatment
Treatment of tuberculosis aims to –
- Interrupt transmission by rendering patients non-infectious
- Prevent morbidity and death by curing patients
- Prevent the emergence of drug resistance
- Prevent relapse.
To achieve the aims, the following strategies are followed –
- Multidrug therapy – Combination of more than one drug for rapid and effective killing of tubercle bacilli
- Short course chemotherapy – Lasting for 6 months (or longer for DR-TB)
Two phase chemotherapy –
The short course chemotherapy is divided into –
- Intensive phase – Aims at aggressive treatment with multiple ATDs that rapidly kill the bacilli making the smear negative, followed by:
- Continuation phase – Aims at killing the remaining dormant bacilli and prevents relapse
DOTS strategy (Directly Observed Treatment, Short course) –
It is recommended by RNTCP and WHO. Here, the strategies used are:
- The entire treatment course is supervised to improve the patient’s compliance
- Treatment response is also monitored by sputum smear microscopy at the end of each phase.
Universal-DST-
It refers to performing drug susceptibility testing (DST) for all TB-patients –
- First performing CBNAAT to determine rifampicin susceptibility; followed by Line probe assay (LPA)
- If found as rifampicin-sensitive, then LPA is performed for other first-line ATDs (e.g. isoniazid)
- If found as rifampicin-resistant, then LPA is performed for second-line agents such as fluoroquinolones (FQs) and second-line injectable (SLI) agent
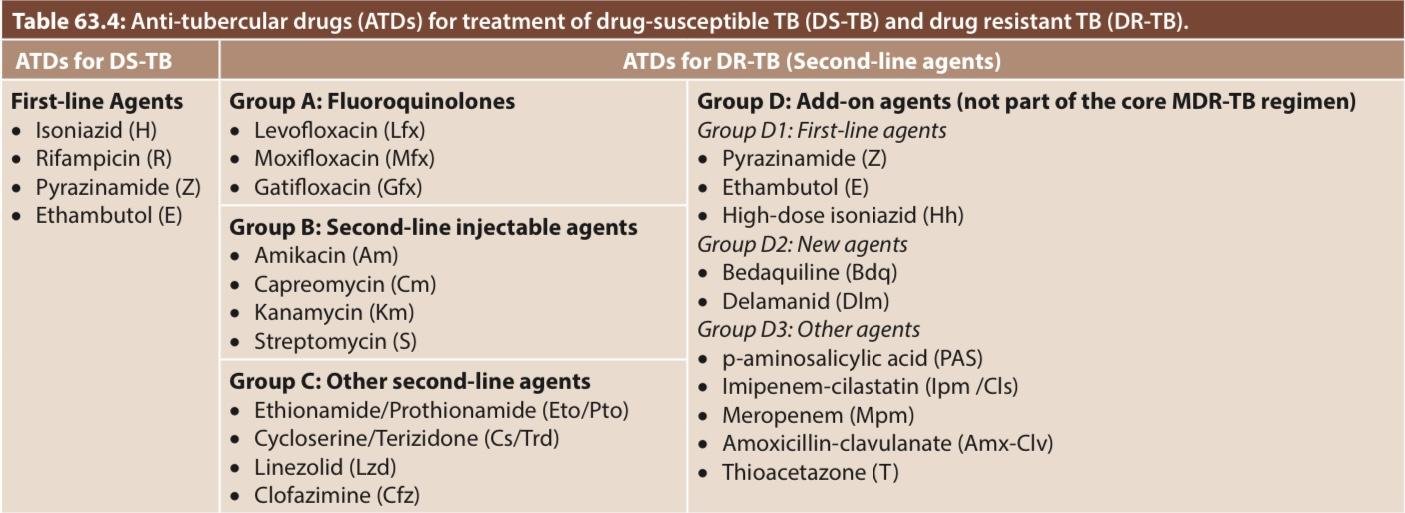
Treatment regimens
The treatment should be planned only after the results of DST are available. The treatment regimens are of various types, depending upon the resistance pattern
Standard regimen for (Drug Susceptible) DS-TB –
- It is a six-month course
- Comprises two phases –
- Intensive phase, with four drugs (HRZE) for two months
- Followed by Continuation phase, with three drugs (HRE) for four months
FDC –
All drugs must be given in fixed dose combination(FDC) tablets as per appropriate weight bands
Daily-oral regimen –
The FDC tablets should be taken orally, once a day.
Regimens for DR-TB
The treatment for DR-TB (Drug Resistance TB) is complex, consisting of use of higher numbers of second-line agents, given for longer duration. The composition of the regimen depends upon the type of DR-TB (Drug Resistance TB)
- Follow-up of treatment: Patients should be followed up at scheduled intervals for the assessment of improvement in clinical and laboratory parameters.
- Clinical follow-up: Should be carried out at least monthly during treatment
- Follow-up laboratory investigation: For PTB (Pulmonary Tuberculosis) cases, sputum smear examination is done at the end of intensive phase. At the end of treatment, sputum smear plus culture is done for every patient.
- Long term follow-up is carried out up to 2 years of completion of treatment
- Follow-up for MDR TB: Sputum smear and culture are performed every month during intensive phase and every three months during continuation phase.

Vaccine prophylaxis Against Tuberculosis
Bacillus Calmette-Guérin Vaccine (BCG)
BCG vaccine was developed by Calmette and Guerin (1921). BCG strain: In India, WHO recommended Danish 1331 strain of BCG is used. It is prepared in Central BCG laboratory, Guindy, Chennai
- Reconstitution of BCG: BCG is available in lyophilized form, should be reconstituted before administration. This is done by using normal saline as diluent. Distilled water is never used as it is irritant. Once reconstituted; it has to be administered within 1 hour
- Administration of BCG: 0.1 mL (0.1 mg TU) of BCG vaccine is administered above the insertion of left deltoid by intradermal route, using a 26 gauge tuberculin syringe
- Phenomena after BCG: If BCG is properly injected intradermally, then the following phenomena develop at the inoculation site:
- After 2–3 weeks: Papule develops
- 5–6 weeks: Shallow ulcer develops, which is covered with crust
- 6–12 weeks: Permanent tiny round scar (4–8 mm diameter) is formed
- 8–14 weeks: Mantoux test becomes positive.
- If overdose is given: The lesion or scar becomes larger and irregular size.
Protection –
- Efficacy – Many trials have shown that BCG has a variable efficacy of 0–80%
- Duration of immunity lasts only for 15–20 years
- Though BCG may not protect from the risk of tuberculosis infection, it surely gives protection to infants and young children against the development of complications such as tuberculous meningitis and disseminated tuberculosis.
Complications –
Most common complications include ulceration at the vaccination site and regional lymphadenitis Rarely, keloid or lupus lesion, and osteomyelitis may develop Very rarely, non-fatal meningitis, progressive tuberculosis and disseminated BCG infection (“BCGitis”) are reported in people with low immunity.
Indications –
- Direct BCG – BCG is directly given to the newborn soon after birth. This strategy is followed by most of the developing countries including India. If not given at birth it can be given later, maximum up to 2 years
- Indirect BCG – BCG is given after performing a tuberculin skin test.
Contraindications –
- HIV-positive child
- Child born to AFB positive mother
- Child with low immunity
- Generalized eczema
- Pregnancy.
Other uses –
BCG induces non-specific stimulation of the immune system; thus provides some protection against certain diseases such as leprosy and leukemia
Chemoprophylaxis
Treatment of selected high-risk tuberculin reactors (i.e. people with latent tuberculosis) aims at preventing active disease. Isoniazid or ethambutol for six months have been tried. However, chemoprophylaxis has several shortcomings such as—(1) it is expensive, (2) risk of developing tuberculosis is minimal in tuberculin reactors, and (3) side effects of the drugs.
Hence, INH preventive therapy (IPT) can be restricted to limited indications such as:
- Adults with HIV who are unlikely to have active TB
- Children with HIV who have no TB symptoms and who are unlikely to have active TB
- All children with HIV who have successfully completed treatment for TB.
#Know more about mycobacteria from “THIS LINK“.
#We Partnered with Achievable.me. Check out their website for awesome study content.


Brillant and updated
All credits go to our author.
Brillant, i need more in Haematology and blood transfussion
Will be coming soon. We are adding more daily…
This is so helpful for me.
Thank you
Thank you for your support. We are proud to have such author in our team. All credits go to her.